Introduction
UM171, a novel compound with hematopoietic stem cells (HPSCs) self-renewal properties has shown great potential for expanding CD34+ HPSCs while preserving their stemness and increasing their engrafting abilities. The compound has been increasingly used for the purpose of CD34+ HPSCs expansion, both in pre-clinical research and in clinical applications. Its use is particularly important in the gene therapy (GT) and bone marrow transplantation (BMT) fields, especially when CD34+ expansion is needed to ensure the success of the therapy.
Recent reports have demonstrated UM171efficacy to expand enriched CD34+ HPSCs from peripheral blood (PB) in osteopetrosis patients, and after mobilization in lymphoma patients.
Here, we demonstrate the feasibility of exploiting the UM171 molecule to directly expand CD34+ HPSCs in bulk PB mononuclear cells (MNCs) without the need of either HPSCs mobilization or CD34+ enrichment.
Material and Methods
Our protocol entails culturing of bulk MNCs directly after Ficoll gradient separation in IMDM media supplemented with 10% FBS, and HPSCs stemness sparing cytokines, used in clinical suitable concentrations: SCF 300 ng/ml, TPO 100 ng/ml, Flt3-L 300 ng/ml and IL3 60 ng/ml +/- 38 nM UM171 for 20 days.
Briefly, quantities of 7.5± 0.5 ml of blood from 2 healthy donors were gradient-separated by Ficoll and yielded a total of 6.6±0.2X10^6 MNCs. Cells were divided in two conditions/donor (Cytokines +/- UM171) and 3.3±0.14X10^6 cell were seeded in 6 well plates at concentration of 1.1X10^6/ml in a total volume of 3 ml. Half of medium was changed every 5 days.
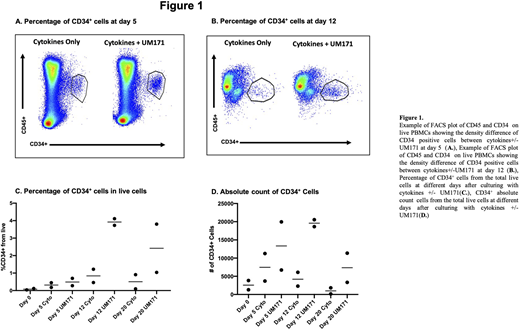
Cell count and phenotype of HPSCs were periodically evaluated to determine the optimal expansion window. Cells were manually counted, and phenotype was measured through Symphony A5 flow-cytometry with this antibody panel: Zombie viability dye, CD45, CD34, and in the last timepoint (day 20) CD11b as an exclusion marker for myeloid differentiation.
Results
UM171 treatment efficiently achieved direct expansion of CD34+ HPSCs in bulk PB MNCs, reaching a peak expansion at day 12 with CD34+ Cells representing 3.9±0.3% of cells (mean±SD of live cells) compared to 0.08±0.04% at baseline (48.75x increase). This increase in CD34+ cell concentration was also partially due to the decline of total PB MNCs cell count during culture time (declined up to 90%). Despite the overall cell count decrease, the CD34+ HPSCs population had an absolute number increase of 6.5±0.916 times with respect to the initial count, peaking at day 12 with an absolute number of 19600±1344 cells compared to 2985±1466 cells at baseline.
If projected to a larger scale, this yield proves the feasibility of obtaining of ∼20X10^6 CD34+ HPSCs from a buffy coat of 350 ml blood donation. This amount would be adequate for a pediatric HSCT, and very handy for multiple experiments with CD34+ HPSCs.
Conclusion
In conclusion, we have provided a proof of concept on the feasibility to directly expand CD34+ HPSCs in bulk PB MNCs. Further characterization of the expanded CD34+ will be needed to ensure stemness, plasticity, and engrafting ability. The advantage of expanding HPSCs in bulk is to preserve the rare naïve CD34+ population that can be lost during the immunoselection process and to take advantage of the myeloid niche, to further enhance the expansion along with UM171.
This expansion approach can be performed at larger scale on the buffy coats often dismissed in blood banks to provide considerable number of CD34+ cells for either banking, research or future clinical application.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.